Short children with a low MUAC respond to food supplementation: an observational study from Burkina Faso
By Fabiansen, C., Phelan, Kevin, P.Q., Cichon, B., Ritz, C., Briend, A., Michaelsen, K.F., Friis, H. and Shepherd, S
Summary of research:
Short children with a low midupper arm circumference respond to food supplementation: an observational study from Burkina Faso. American Journal of Clinical Nutrition, February 2016, vol. 103, no. 2, pp.415-421.
Location: Burkina Faso
What we know: Where MUAC is used as an admission criterion for SAM treatment, it is common practice to exclude children with lengths <67cm.
What this article adds: An observational study, nested in a randomised trial, compared the growth rate between 468 moderately malnourished children <67 cm and ≥67 cm in length, admitted to a supplementary feeding programme (SFP) solely under MUAC criterion (115-124 mm). Weight gain was 12% during 12 weeks of supplementation and MUAC increased 6% (10-12 weeks) and did not differ between short and long children. Weight-gain velocity was similar between both groups from baseline; MUAC-gain velocity was comparable from week four. Short children took longer to recover; 71% short v 90% long had recovered by week 12. The authors suggest to admit children <67cm who fulfil MUAC criteria for admission to SFPs.
Moderate wasting alone affects 5% of children under five years old worldwide and is associated with a three-fold increased risk of death. Moderate wasting is usually defined as a weight-for-height Z score (WHZ) between –3 and –2, and/or a measure of mid-upper arm circumference (MUAC) between 115 and 124 mm. MUAC is a simpler assessment tool and may be better than WHZ in identifying children at risk of death. The management of children with moderate acute malnutrition (MAM) relies primarily on food supplementation in outpatient programmes. In such programmes, MUAC is often used as the sole admission criterion where it is common practice to exclude from treatment children with lengths <67cm.
These short children with a low MUAC are believed to be either young (<6 months) or stunted, rather than acutely malnourished, and therefore would not have the rapid “catch-up” growth typical of more wasted children. A secondary concern is that they may be harmed by prolonged exposure to supplementary foods, for example through excessive gain of fat mass. The World Health Organisation (WHO) has called for research to identify the appropriate MUAC admission and discharge criteria for children <67cm and aged ≥ 6 months. This study aims to contribute to this evidence base by testing the hypothesis that, among children given supplementary feeding based on an MUAC of 115-124 mm as the sole criterion, there would be no difference in growth rate between children <67 cm and those ≥67 cm in length at programme admission.
Study overview
This was an observational study nested in a randomised trial (Treatfood1) that investigated the effectiveness of new, improved formulations of corn-soy blend (iCSB) supplement and lipid-based nutrient supplements (LNS). Six iCSBs and six LNS combine different soy qualities (dehulled soy, soy isolate) and different amounts of dried skimmed milk. The study on the different product formulations found that all were well accepted. (Iuel-Brockdorf, Draebel, Ritz et al, 2016). The study was conducted in the Province du Passoré in the northern region of Burkina Faso in an area qualifying for supplementary feeding programmes (SFPs) based on the prevalence of acute malnutrition. Children were randomly assigned to 12 different groups (six based on iCSB and six based on LNS) and given supplements with an energy amount of 500 kcal per daily serving (130g iCSB or 92g LNS) for a three-month period. Children aged six to 23 months with a MUAC of between 115 and 124mm but with WHZ ≥-2 were included. This cohort was divided into two groups by length at admission: 67 cm (“short”) and ≥67 cm (“long”).
Children were scheduled for a clinic visit every two weeks during the 12-week supplementation period, during which anthropometric and clinical status were assessed, including MUAC, weight and height/length. Anthropometric measurements were performed by trained staff, after standardisation sessions. Increases in weight and MUAC were calculated as percentages from baseline. Weight-gain velocity was de?ned as a change in weight (in g/kg/d; per milled (%) change/d) from baseline. MUAC-gain velocities were calculated similarly as change/d (i.e., 0.01 mm/cm/d).
Recovery was de?ned as a MUAC measurement >125 mm on two consecutive visits (two weeks apart) as applied in programmes. Children who missed scheduled visits were visited by community health workers at their homes and encouraged to return for follow-up. Children who missed two consecutive visits were regarded as defaulters. Children received vitamin A supplementation, deworming and medical treatment or referrals based on the integrated management of childhood illness (IMCI) guidelines. Linear, mixed-effects models and regression models were used to compare gains in weight and MUAC, adjusted for intervention, season, sex, age and site.
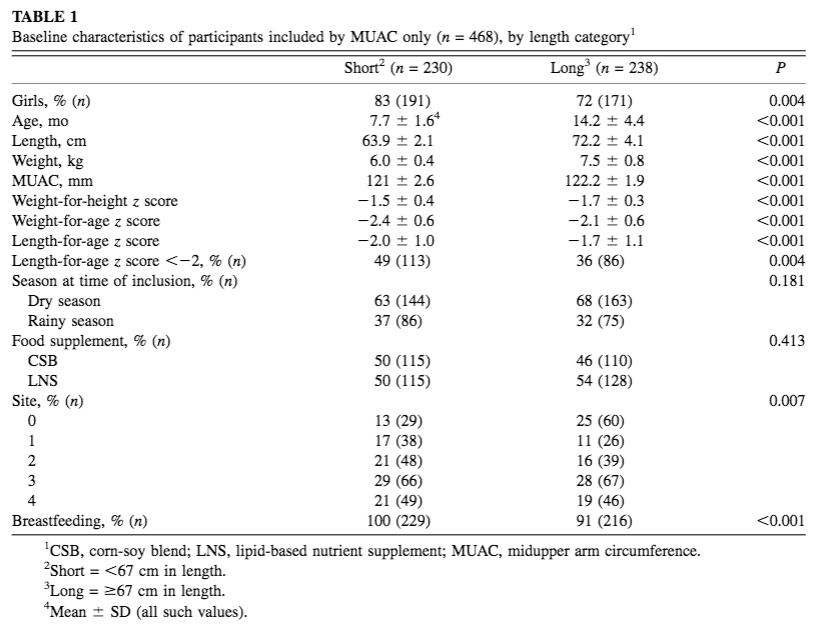
From 9 September 2013 to 29 August 2014, a total of 1,609 children were included in the Treatfood trial. Fifty per cent of children (804) were included by both WHZ and MUAC criteria (this high degree of overlap is expected in younger/shorter children), 21% (337) by WHZ alone and 29% (468) by MUAC alone. Among the 468 children recruited by MUAC-only, 230 (49%) were <67cm (short) and 238 (51%) were ≥67cm (long). Baseline characteristics of MUAC-only admissions by length category are included in Table 1. The average age of MUAC-only short children was 7.7 ±1.6 months; average length was 63.9 ± 2.1 cms; average weight was 6 kg ± 1.4; and most (83%) were girls.
Results
After 12 weeks of supplementation, weight gain was 12% from baseline and did not differ between short and long children. The increase in MUAC was 6% after ten to 12 weeks of supplementation and did not differ between groups. Weight-gain velocity from baseline showed a similar development between groups throughout supplementation. MUAC-gain velocity from baseline was also similar between groups from week four. The longer children gained in MUAC more quickly than they did in weight over the first two weeks of the study, compared to the short children. But both groups were gaining weight and MUAC at a faster rate than is described by the WHO 2006 growth reference. Weight and MUAC gain velocities from the last visit remained similar between short and long children; initially high, they were approaching velocities for well-nourished reference children towards the end of supplementation. There was no effect modification by different product formulations (12 in total) on weight-gain velocity (P = 0.65) or MUAC-gain velocity (P = 0.45).
Table 1: Baseline characteristics of participants included by MUAC only (n = 468), by length category
There was a weak overall difference in weight-gain velocity from baseline to 12 weeks between quartiles of baseline length (P = 0.03), reflecting a slight downward trend from the lowest to the highest quartile. Weight-gain velocity across length quartiles was not modified by stunting (P= 0.32). For MUAC-gain velocity, there was no overall difference between length quartiles (P = 0.12) and no effect modification by stunting (P = 0.75). The short group took more time to reach the MUAC recovery criterion than did the long group: at week four, 39% of short compared with 57% of long children had recovered (P = 0.01). This difference of around 20% points was maintained throughout supplementation, such that at week 12, 71% of children in the short group had reached recovery, compared with 90% of children in the long group. At the last visit, week 12, an additional 10% of short children and 3% of long children reached a single MUAC measurement of ≥125 mm. More than half of the children in the long group had recovered according to MUAC at week two in the study, whereas for the short group this occurred at week six in the study.
The authors conclude that there was no evidence of a difference in percentage of weight gain or weight-gain velocity during supplementary feeding in short or long children aged six-23 months. On this basis, the authors suggest a policy change to include children <67 cm in SFPs if their MUAC is between 115 and 124 mm and their WHZ is ≥-2.
References
1 For trial information, see link here
Iuel-Brockdorf A., Draebel T.A., Ritz C., Fabiansen C., Cichon B., Christensen B. V., Yameogo C., Oummani R., Briend A., Michaelsen K.F., Ashorn P., Filteau S., Friis H., 2016. Evaluation of the acceptability of improved supplemental foods for the treatment of moderate acute malnutrition in Burkina Faso using a mixed method approach. Appetite Vol. 99 April 2016 pp34-45.