Infant formula advertising in medical journals: a cross-sectional study (and struggle to publish)
By Sarah Morgan, Tony Waterston and Marko Kerac
Sarah Morgan is a Public Health Registrar currently working with National Health Service (NHS) England in immunisation and screening. She has worked in global child health, nutrition and TB with Medair, World Vision UK and as chair of the board of Target TB. This article is based on initial research carried out as part of her MSc in International Child Health at University College London (UCL).
Tony Waterston is a retired consultant paediatrician with six years’ experience working in Africa. He led the Royal College of Paediatrics and Child Health health diploma programme in Palestine between 2000 and 2015 and is an honorary Fellow of the College. He is also lead moderator of Child Health Information for All (CHIFA).
Marko Kerac is Assistant Professor in Public Health Nutrition & Programme Director of the MSc in Global Health Nutrition at the London School of Hygiene and Tropical Medicine. He was a National Institute for Health Research Clinical Lecturer in Public Health at UCL at the time this project was originally conducted.
Marko Kerac acknowledges NIHR support for his UCL Academic Clinical Lecturer post at UCL from 2012-2014.
Location: Global
What we know: Breastmilk substitute (BMS) marketing within the health system, combined with other factors, can undermine breastfeeding.
What this article adds: The extent of BMS advertising in leading medical journals was examined from 2003-2012 and compliance of 2012 adverts was assessed against the International Code of Marketing of Breast-Milk Substitutes (‘the Code’). Although BMS advertising was uncommon overall (in 12 journals reviewed, 8.6% of pages were advertisements of which 1.7% were for BMS), it varied markedly between different journals (only five out of 12 journals carried BMS adverts at all; one publishing group was responsible for almost 75% of all BMS advertising). Code compliance was poor: all advertisements contained purely promotional statements and none contained all of the information and warnings about BMS stipulated in the Code. Possible reasons for this are discussed. Journals should either screen BMS advertisements for Code compliance or, ideally, not carry BMS adverts at all; independent sources of information should be promoted instead.
Background
Globally, 12% of all deaths of children aged under five years old are attributed to sub-optimal breastfeeding (Black et al, 2013). Infants in high-income countries fed breastmilk substitutes (BMS) have greater risk of gastrointestinal illness, otitis media and lower respiratory tract infections (Ip et al, 2009). The reasons for low breastfeeding rates are multi-factorial, but include the inappropriate marketing of BMS by manufacturers (WHO, 1981, 1998). An International Code of Marketing of BMS and subsequent relevant World Health Assembly (WHA) Resolutions (collectively known as ‘the Code’) aims to protect infant feeding from commercial influence. Despite the Code, questionable BMS marketing practices have been noted by several studies, including in healthcare settings (McInnes et al, 2007; Sadacharan et al, 2011; Taylor, 1998). Advertisements for BMS, including those in medical journals, should comply with stipulations of the Code. This states that information “should be restricted to scientific and factual matters, and such information should not imply or create a belief that bottle feeding is equivalent or superior to breast-feeding” and must include relevant warnings about BMS. This study aimed to describe the extent and quality of BMS advertising in high-impact paediatric and general medical journals.
Methods
A cross-sectional study was carried out by hand-searching high-impact paediatric and general medical journals published between 2003 and 2012 to quantify the prevalence of BMS advertising. Two investigators also independently assessed the content of advertisements published in 2012 for the presence of purely promotional statements and certain information statements stipulated by Articles 7.2 and 4.2 of the Code.
Findings
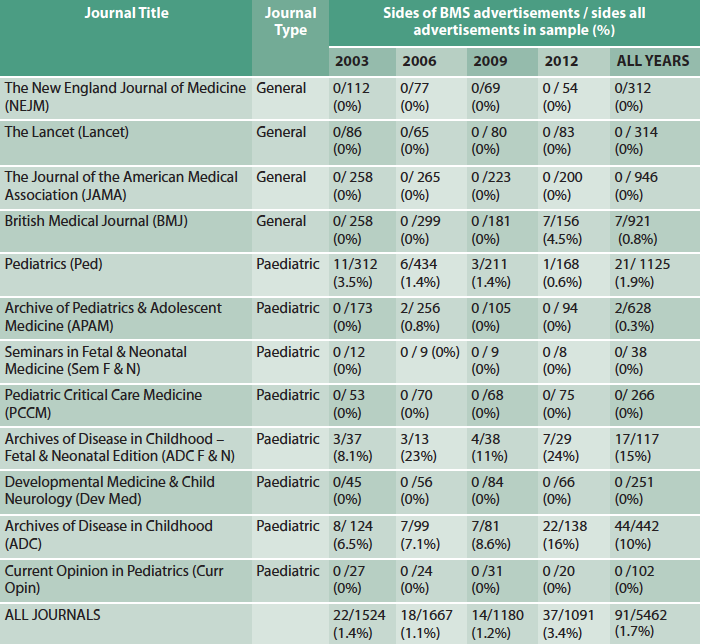
Of 63,167 pages searched in 12 journals (eight paediatric, four general), 5,462 (8.6%) were advertisements and 91/5462 (1.7%) of these were for BMS. Some journals carried no BMS adverts at all (Table 1); of five journals carrying BMS adverts, four were paediatric journals; one publishing group was responsible for almost 75% of all BMS advertising.
Table 1: BMS advertising as proportion of total advertising (in sides)
Five types of BMS products for infants under six months were advertised, with some advertisements simultaneously promoting multiple types. The most common advertisements were for allergy or intolerance milk (27 of 91 sides; 30% of BMS advertising), preterm milk (23 of 91 sides; 25% of BMS advertising) and general first milk (18 or 91 sides; 20% of BMS advertising). A much smaller percentage of advertisements were given to high-energy or catch-up milks (6/91 sides; 7% of BMS advertising) and comfort milks (4/91; 4% of BMS advertising).
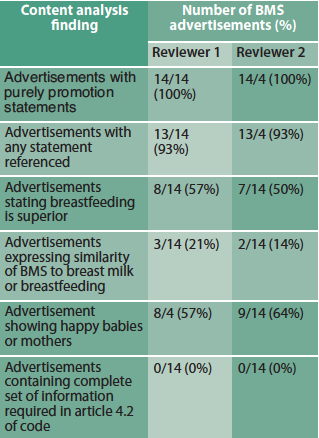
The 2012 sample contained 39 BMS advertisements, 14 of which were unique adverts. The reviewers agreed that all advertisements met the study definition of containing purely promotional statements and none contained all the information and warnings about BMS stipulated in Article 4.2 of the Code (Table 2).
Table 2: Content results for 2012 BMS advertisements
Discussion
Although uncommon overall, in this study period BMS advertising was carried by leading clinical journals and, in particular, two leading child health journals, Archives of Diseases in Childhood (ADC) and ADC Fetal and Neonatal edition. Adverts also appeared in the British Medical Journal (BMJ), which had the highest print circulation in the UK (BMJ Group, 2013). BMS advertisements, therefore, have the potential to reach and influence many health professionals, especially those working in paediatrics.
Our second major finding of poor advert compliance with the Code is thus an important concern, given the documented deficiency in health professionals’ knowledge regarding breastfeeding (Brodribb et al, 2008; Freed et al, 1995) or the risks of BMS (Feldman-Winter et al, 2008; Schanler et al, 1999) and health professionals’ use of company materials for information, especially for specialist milks (McInnes et al, 2007).
Recommendations and conclusions
We see two possible ways forward. At a minimum, journals should do more to screen and review BMS advertisements to ensure that they are fully compliant with the Code. A far better recommendation is that journals do not carry BMS adverts at all. The rationale for BMS advertising as an information source is questionable, given that there are many other opportunities for health professionals in the UK to access independent and comparative information about BMS products (Crawley & Westland, 2013).
In conclusion, it is encouraging that most journals carry limited/no BMS advertisements; however it is regrettable that existing adverts are poorly compliant with the Code. While health professionals need information on BMS, especially specialist BMS products, advertisements are unlikely to drive evidence-based best practice. Instead, an expansion of alternative sources of independent, in-depth information is needed, which would better empower health professionals to protect breastfeeding and, where BMS are indicated, to inform choices between the large variety of BMS available more accurately. Future research is needed to investigate how today’s journals compare with those reviewed in this study.
A final word: the struggle to publish
This project was originally carried out as an MSc project at UCL (SM). When we initially presented findings at UK conferences they were positively received, winning an oral presentation prize at one meeting. Publication has been more challenging, with our paper initially rejected by two of the journals which carried the most advertisements. While we acknowledge the limitations of our work (e.g. a limited number of journals reviewed; limited years sampled over our ten-year timeframe; an unavoidable element of subjectivity in assessing Code compliance; debates about how specialist milk advertising should differ from general BMS), we were surprised by the reason given by a final journal we submitted to. Despite editors sending our paper for review and (we believe) us addressing the relatively minor reviewer comments, we were unexpectedly told at the last stage that the paper was “beyond journal scope” – in spite of other articles on breastfeeding having been published previously. We wonder whether this in itself tells a story regarding the many complex issues around BMS marketing.
In 2016 the UK Royal College of Paediatrics and Child Health (RCPCH) voted to continue to accept funds from infant formula companies (Thornton, 2016), triggering important discussions as to the pros and cons of this stance (Costello et al, 2017). To what extent this vote might have been influenced by the ‘normalisation’ of BMS advertising in journals is of course unknown, but it is certainly one of many possible factors. Of note, ADC and ADC Fetal and Neonatal, the top two journals advertising BMS in our sample, are official journals of the RCPCH and go out regularly to all members and fellows of the College. In future research, we would be interested to review how many other professional associations (e.g. for midwives, dieticians, nurses and others with front-line patient contact) also carry similar advertising in member journals.
For more information, please contact Sarah Morgan.
References
Black, RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Uauy R. (2013). Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet, 382(9890), 427–451. doi: 10.1016/s0140-6736(13)60937-x
BMJ Group. (2013). Print Advertising Opportunities 2013. Retrieved 01.07.2013 from http://group.bmj.com/group/advertising/Print%202013%20Media%20Pack.pdf
Brodribb W, Fallon A, Jackson C & Hegney D. (2008). Breastfeeding and Australian GP registrars – their knowledge and attitudes. J Hum Lact, 24(4), 422-430. doi: 10.1177/0890334408323547
Costello A, Branca F, Rollins N, Stahlhofer M & Grummer-Strawn L. (2017) Health professional associations and industry funding. The Lancet, Volume 389, Issue 10069, 597–598,
Crawley H & Westland S. (2013). Infant milks in the UK. A practical guide for health professionals. UK: First Steps Nutrition Trust.
Feldman-Winter LB, Schanler RJ, O’Connor KG & Lawrence RA. (2008). Pediatricians and the promotion and support of breastfeeding. Arch Pediatr Adolesc Med, 162(12), 1142-1149. doi: 10.1001/archpedi.162.12.1142
Freed GL, Clark SJ, Sorenson J, Lohr JA, Cefalo R & Curtis P. (1995). National assessment of physicians’ breast-feeding knowledge, attitudes, training, and experience. JAMA, 273(6), 472-476.
Ip S, Chung M, Raman G, Trikalinos TA & Lau J. (2009). A summary of the Agency for Healthcare Research and Quality's evidence report on breastfeeding in developed countries. Breastfeed Med, 4 Suppl 1, S17-30. doi: 10.1089/bfm.2009.0050
McInnes RJ, Wright C, Haq S & McGranachan M. (2007). Who’s keeping the code? Compliance with the international code for the marketing of breast-milk substitutes in Greater Glasgow. Public Health Nutr, 10(7), 719-725. doi: 10.1017/s1368980007441453
Sadacharan R, Grossman X, Sanchez E & Merewood A. (2011). Trends in US hospital distribution of industry-sponsored infant formula sample packs. Pediatrics, 128(4), 702-705. doi: 10.1542/peds.2011-0983
Schanler RJ, O’Connor KG & Lawrence RA. (1999). Pediatricians’ practices and attitudes regarding breastfeeding promotion. Pediatrics, 103(3), E35.
Taylor A. (1998). Violations of the international code of marketing of breast milk substitutes: prevalence in four countries. BMJ, 316(7138), 1117-1122.
Thornton, J. (2016). Paediatricians vote for college to continue accepting funds from infant formula companies. BMJ, 355, i5827. doi: 10.1136/bmj.i5827
WHO. (1981). International Code of Marketing of Breast-milk Substitutes. Geneva WHO.
WHO. (1998). Evidence for the ten steps to successful breastfeeding. Geneva: WHO.